Certificate of Analysis
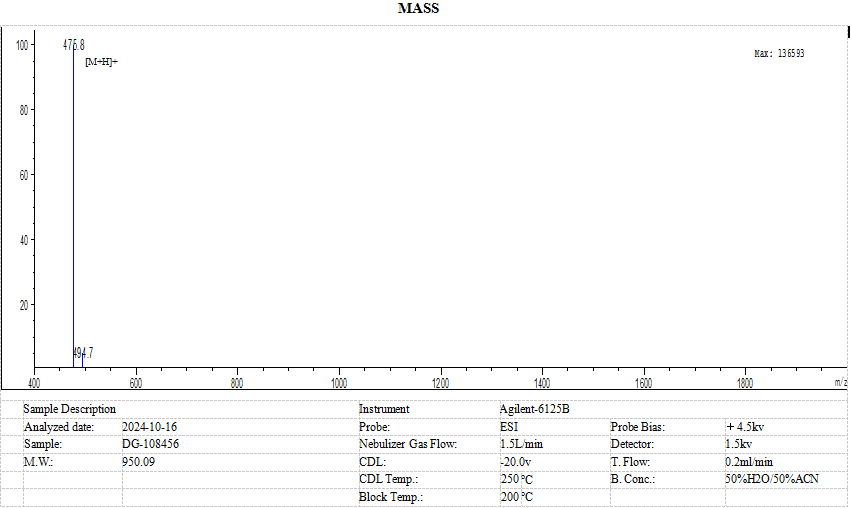
The certificate of analysis for custom syntheses contains the following information: Name, Product Number, Lot Number, Theoretical MW, Sequence, Mass (MS), Amino Acid Analysis, HPLC Profile, Peptide Purity, Peptide Content and Counterion.
The certificate of analysis for catalogue products contains the following information: Name, Lot Number, Theoretical MW, Sequence, Amino Acid Analysis, HPLC Profile, Peptide Purity, Peptide Content, Counterion, Details of Solubility. The identities of catalogue peptides are checked by cochromatography with internal house standards whose own identity has already been proven by mass spectrometry.
The certificate of analysis for peptide generics can be obtained fromDGpeptides Co., Ltd. on request. Usually, the specifications will comply with current US and European pharmacopoeias.
Peptide Purity
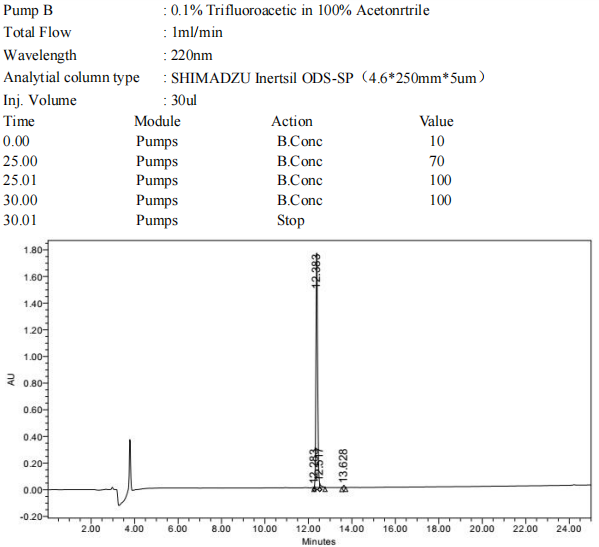
"Peptide purity” is not synonomous with .peptide content. Peptide content provides information about the relationship of the peptide to other components in the lyophilisate. Peptide purity refers to the relative purity of the main product to other peptide impurities (when detectable). Peptide purity is calculated as the area percentage of UV-positive (210-220 nm) material eluting under the main peak of the HPLC chromatogram.
Peptide Content
Most peptides contain basic and acidic side groups obligating the presence of counterions (normally trifluoroacetate). Because very few peptides crystallize, the normal method of preparation involves a final lyophilization step.Thus, in addition to the peptide and counterion, the lyophilisate contains up to 5% residual moisture.
Unless otherwise stated, given quantities of peptides (nominal quantity on label) refer to the quantity of peptide lyophilisate (peptide + counterion + moisture) in the vial.
Before reconstituting the peptide, ensure that you have taken the actual net peptide content given in the certificate of analysis into consideration. The peptide content for most peptides usually lies in the range of 75 - 85%, but can be as low as 50 - 60% in short peptides with an excess of basic amino acid residues.
Additional analyses
In addition to the data provided on the certificate of analysis, a considerable number of additional tests can be performed on request. Most of these will be performed externally and charged as extra positions. Tests include: MS-MS, Sequence Analysis, Chiral Analysis, GC-MS Racemate Determination, Optical rotation, Acetate Determination, TFA Determination, Moisture Determination, Residual Solvent Determination.
HPLC Analysis Report